19 Predict Which of the Following Has an Ionic Bond
N to N 30-3. A Sodium metal and liquid bromine _____.
Ionic compounds have high.

. The ions in a crystal lattice are arranged randomly and the overall charge is zero E. Problem 419 Write the formulas of the following ions. Thats definitely an Ionic bond.
Predicting and Naming Binary Ionic Compounds with Transition Metals Classwork 37. Ionic compounds have high melting points B. Most of these solids are soluble in H 2 O and conduct electricity when dissolved.
E none of the above. 2 has ionic bonds. A Cl 2CO b MnO c NCl 3 d CoBr 2 e K 2S f CO g CaF 2 h HI i CaO j IBr k CO 2 Solution.
Want to see the. Write the Lewis structure and chemical formula of the compound with a molar mass of about 70 gmol that contains 197 nitrogen and 803 fluorine by mass and determine the formal charge of the. What is the formula of the compound that occurs when Strontium and Chlorine.
Chemistry questions and answers. Which of the following statements about ionic compounds are true. Ionic compounds formed from the following combinations of elements.
A NiCl 2 SOLUBLE most chlorides are soluble b Ag 2S INSOLUBLE most sulfides not soluble c Cs 3PO 4 SOLUBLE while most phosphates are not soluble Grp IA Cs is d SrCO 3 INSOLUBLE most carbonates not soluble e PbSO. A H 2 S B NH 3 C CH 4 D all of the above E none of the above. Polar covalent bonds c.
Ionic compounds can be solid liquid or gas at room temperature C. First week only 499. Predict which of the following has an covalent bond.
A SO 2 b CaF 2 c N 2 H 4 d Al 2 SO 4 3. 3 b sulfur dioxide SO. For the elements Rb F and O the order of increasing electronegativity is.
D all of the above. Predict which of the following has an ionic bond. First week only 499.
19 ______ A SnO2 B CO2 C SO3 D all of the above E none of the above. This is opposed to an ionic bond where electrons are actually transferred from one atom to another. D all of the above.
Between zero and 18 is olar covalent. When electronegativity difference is 18 or. And so then theres also polar Co Vaillant with the difference in electro negativity of about 04 aluminum and oxygen aluminum sometimes considered a metal Lloyd.
Predict if ions the following elements will produce Anions or Cations or neither. A SF 6 B H 2. 6 Predict which of the following has an ionic bond.
The ability to conduct electricity in solution is why these substances are called electrolytesTable salt NaCl is a good example of this type of compound. D all of the above. Solution for Using the periodic table predict whether the following compounds are ionic or covalenta SO2b CaF2c N2H4d Al2SO43.
Weve got the study and writing resources you need for your. Start your trial now. We usually call those above 18 ionic but those very close to 18 or 19 still are polar covalent although more ionic than covalent.
Bond Polarity and Electronegativity. A Alkaline Earth metals B Halogens C Chalcogens D Alkali metals E Transition metals Predicting and Naming Binary Simple Ionic Compounds 20Which of the following compounds would you expect to be ionic. Predict which of the following compounds are ionic and which are covalent based on the location of their constituent atoms in the periodic table.
Choose the compound with the most ionic bond. Is kJmol 1e 1602x1019C d is the meter and k 899X109 JmC2-. Start your trial now.
These electrons are simultaneously attracted by the two atomic nuclei. Start studying Chem 2A Ch. A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms.
D TUNICUL LICO 30 30 Predict which of the following has an ionic bond. At the macroscopic scale ionic compounds form lattices are crystalline solids under normal conditions and have high melting points. Covalent bonds occur between identical.
Most ionic compounds are insoluble in water D. This problem has been solved. 419 Using solubility guidelines predict whether each of the following compounds is soluble or insoluble in water.
Predict whether the following compounds are ionic or covalent. Solution for Predict the empirical formulas of the ionic compounds formed from the following pairs of elements. Predict whether each of the following is held together by ionic or by covalent bonds.
Predict whether each of the following is held together by ionic or by covalent bonds. - Ba and I. Rb F O b.
You can remember the. E none of the above. 5 Predict which of the following has an ionic bond.
D TUNICUL LICO 30 30 Predict which of the following has an ionic bond. And predict the approximately where the ionization energy of xenon is likely to fall. Bond is likely to be ionic or covalent.
What is the formula of the compound that occurs when. One way to predict whether a bond is ionic or covalent is to look how far apart the two atoms forming the bonds are in the periodic table. When electronegativity difference is greater than 04 and less than 18 then bond between the two atoms is a polar covalent bond.
If one atom is on the far left Group 1 or 2 and the other is on the far right Group 5 6 or 7 then the atoms will have large differences in EN and will form an ionic bond. But we think of it as a metal with oxygen thats a non metal. Learn vocabulary terms and more with flashcards games and other study tools.
But theres a difference in electro negativity so it would be polar Co. 4 Predict which of the following has an ionic bond. 09-3 Orbitals Consistent with Molecular Shapes.
A covalent bond is formed when atoms share one or more pairs of electrons. A aluminum oxide Al. Atoms having greatly differing electronegativities are expected to form.
93 Bonding in Ionic Compounds. Nonpolar covalent bonds d. 3 b magnesium nitride Mg.
Predict which ionic compound has the higher melting point in each of the following pairs a NaCl and NaBr b NaCl and KCl. Take the difference of electronegativity between the two atoms. A AlCl3 B Cu2s C FeF3 D all of the above E none of the above.
96 Exceptions to the Octet Rule. Dec 3 2012. If delta EN electronegativity 18 or 19 that is about 50 ionic50 covalent.
Predict which of the following compounds are ionic and which are covalent based on the location of their constituent atoms in the periodic table.

The Ionic Bond Boundless Chemistry

Ionic Bond Examples Biology Dictionary

Ionic Bond Examples Biology Dictionary
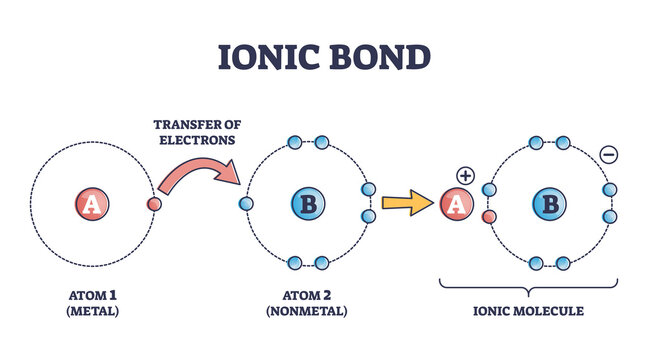
Ionic Bond Images Browse 795 Stock Photos Vectors And Video Adobe Stock
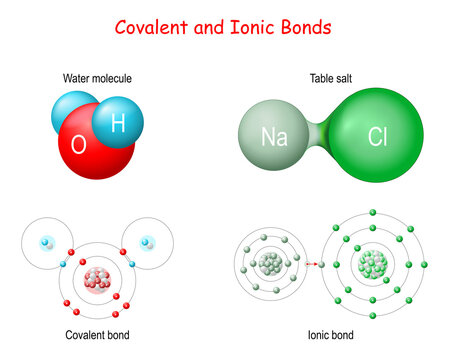
Ionic Bond Images Browse 795 Stock Photos Vectors And Video Adobe Stock

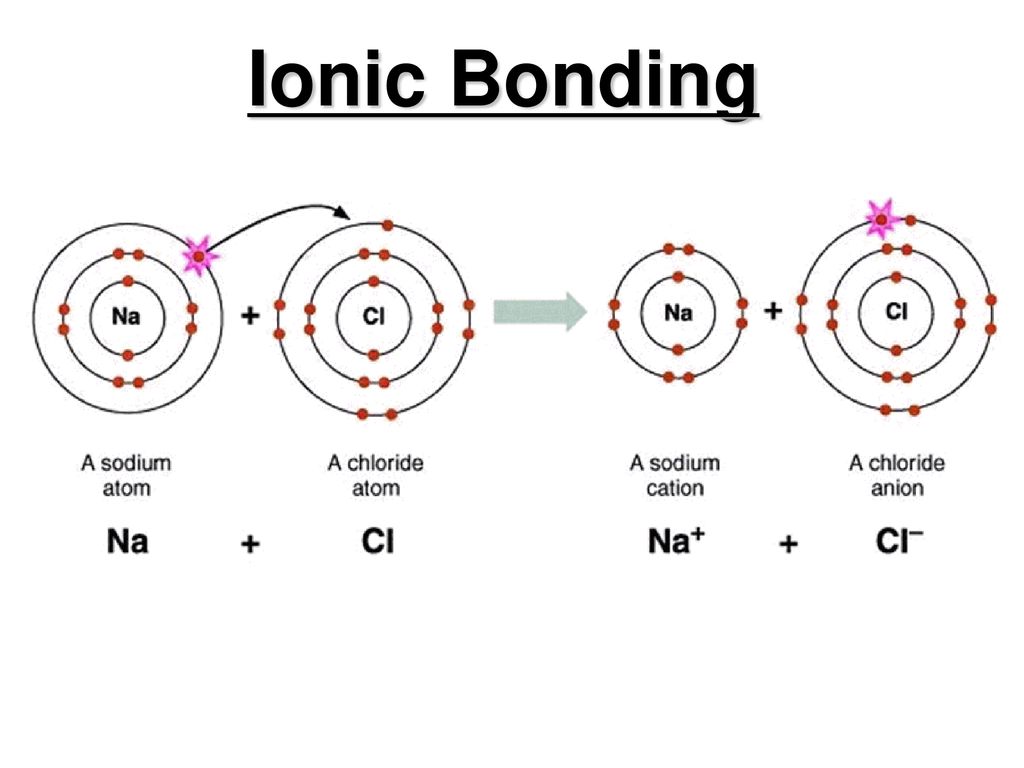
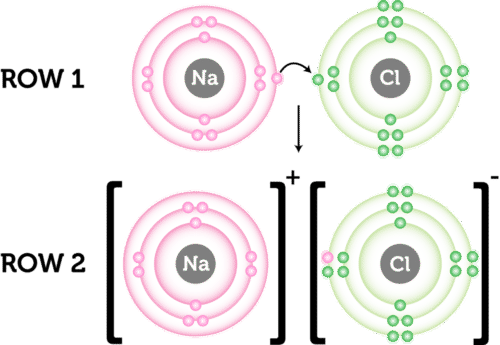


Comments
Post a Comment